

| Rock types | | | Properties | | | Volcanoes | | | Arcticles |

Crystallography is a huge topic and in-depth coverage of this subject is far beyond the scope of this site.
Here we have included basic definitions of common crystal forms with some examples. It is important to note that minerals with the same chemical structure can show remarkable differences at the crystal level.
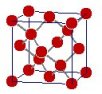
Probably the most extreme example being diamond and graphite. The crystal structures of graphite and diamond are shown below. The graphite consists of hexagonal sheaths which are parallel and do not have chemical bonds between them.
As a result the graphite is soft, brittle and easily breakable. Diamond, on the other hand, is a cube with an octahedral cleavage. It has strong chemical bonds between all carbon atoms, ensuring rigid and perfect crystals.
 | GRAPHITE |
 | DIAMOND |
Common crystal forms
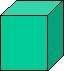
| Cubic contains three axes of equal length. All axes are positioned at right-angle to each other. A cube is also referred to as a closed form and can itself be a crystal. | ||
 |
| |
| Orthorhombic contains three axes which are of different lengths. All axes are positioned at right-angles to each other. | ||
 |
| |
| Monoclinic contains three axis of unequal length. Two axes are not at right angles. The third axis is at a right angle to the plane containing the other two axes and it is often referred to as the 'symmetry axis'. | ||
 |
| |
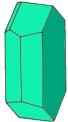
| Triclinic contains three axes. All axes are of different length and none at a right angle to the others | ||
 |
| |
| Trigonal contains four axes with three of equal length, all three arranged in the horizontal plane. The forth axis is perpendicular to that plane and is of a different length to the others. Axes and angles in this system are similar to the Hexagonal System. In the cross-section of a Trigonal crystal there will be three sides. | ||
 |
| |
| Hexagonal contains four crystallographic axes consisting of three equal horizontal, or equilateral axes at 120 degrees to each other, as well as one vertical axis which is perpendicular to the other three. This vertical axis can be longer or shorter than the horizontal axes. | ||
 |
| |
© Biscuit Software 2016