
July Editorial
The secret life of inner amber.

July Editorial
The secret life of inner amber.
|
|
Amber has been appreciated by humans for at least 13,000 years. It was used for ornamentation by stone-age cave-dwellers and it remains popular in jewellery shops today. While found in archaeological sites across Europe, some of the finest Amber ornaments have been discovered in tombs in Mycenae. In the second millennium BC, Mycenae was one of the major centres of Greek civilization, where the heroes of legend are reputed to have gathered before sailing to the Trojan war. Now Mycenae is an archeological site located some 90 km from Athens, and amber jewellery based on Mycenaean originals is enjoying a fashion renaissance. |
|
|
Amber is an unusual jewelstone because unlike almost any other, it is organic in origin. Amber is sometimes poetically called the 'tears of the sun' from the legend of the daughters of the sun-god who became trees and wept golden resin. Amber is indeed created from tree resin, but much of it fossilized during the Cretaceous age some 66 million years ago. The hardness varies from 1-3 on the Mohs scale, depending on the type of Amber and its age. It is thought that younger Amber is softer because it has had less time to fossilize. In any case, the different classes of Amber (see below) also have different hardnesses. In colour, Amber ranges from white and pale yellow to orange, red, brown or even green or bluish black. Because of its organic origin, the structure of amber is amorphous - which means that it lacks the orderly arrangement of atoms seen in minerals. Amber is mostly classified by the plants which produced the original resin. Each species of plant produces its resins through a unique blend of chemicals, the composition of which is highly variable. All however have terpenes, which make up large and diverse class of organic compounds which link up as the resin matures. How the internal matrix of this compound joins itself into a coherent structure is largely unknown. One of the challenging areas for those studying Amber is to determine why and how the chemical and structural composition came about. To unravel some of Amber's internal secrets, Jennifer Poulin and Kate Helwig of the Canadian Conservation Institute looked into the role played by succinic acid in the internal structure of certain Ambers. The material studied belongs to the most abundant class of Amber, namely Class I . Succinic acid is present in Ambers of Class Ia and Id but not in other sub-groups. The best-known Amber which contains succinic acid is 'normal' Baltic Amber. Baltic Amber is actually so distinguished by its high yield of succinic acid (3-8%) that it is often referred to as succinite. Succinite is also one of the hardest of Ambers ranking at between 2-3 on the Mohs scale. Succinic acid, historically known as 'spirit of amber', is a white, odorless solid with the chemical formula C4H6O4 and structural formula HOOC-(CH2)2-COOH. Succinic acid was originally obtained by pulverising Amber and distilling it in a sand bath (A sand bath is a common piece of laboratory equipment consisting of a container filled with heated sand. This equipment is used to provide even levels of heat for another container, usually during a chemical reaction.). 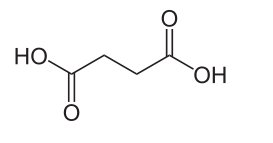 Previous attempts at investigating the internal structure of amber used a technique called pyrolysis. Pyrolysis is the thermochemical decomposition of organic material at elevated temperatures in the absence of oxygen. With Amber this approach was largely unsuccessful, because unfortunately the technique leads to irreversible changes in chemical composition and physical phase. So in their most recent studies Jennifer Poulin and Kate Helwig used a novel gas chromatographic methodology also known as pyrolysis-gas chromatography-mass spectrometry. By this method of chemical analysis, a sample is heated until it decomposes to produce smaller molecules. These are then are separated by gas chromatography and the type of molecule is detected using mass spectrometry. By slowing down the rate of heating and prolonging the process of pyrolysis, Jennifer Poulin and her colleague managed to elute unbroken succinyl ester cross-linkages from the amber samples. These results provide direct molecular evidence that the key role of succinic acid within Class Ia and Class Id resinite is to cross-link the macromolecular structure. And this cross-linking is what gives stability and durability to the Amber's chemical structure. It is postulated that other materials must play the same role in Amber types which lack succinic acid, but this remains unknown. The 'tears of the sun' still keep many of their ancient mysteries, but at least one puzzle has been cracked. Journal Reference: | |
| _______________________________ | ||||
| Home | | | Shopping | | | Database |
© Biscuit Software 2004-2015
All rights reserved