July Editorial
'Superhard graphite'

July Editorial
'Superhard graphite'
|
On the one hand we have graphite. It is very soft, which is why we use it in pencils, and it has a stable structure under normal conditions. This structure is composed of multiple layers of flat planes with a distance of 0.335 nm between the planes. Each layer of graphite has carbon atoms arranged in a hexagonal lattice with separation of 0.142 nm. This crystal structure makes graphite very soft and flaky and good for drawing with (the name of the material comes from ‘grapho’ the Greek for ‘draw’.) Graphite is gray/black in colour and interestingly it is a good conductor of electricity.
|
On the other hand we have diamond. Diamond is very hard, transparent and won’t conduct electricity at all. Speaking crystallographically, diamond is a cube with an octahedral cleavage. It has strong chemical bonds between all carbon atoms, which ensures rigid and perfect crystals. Diamond is formed under high pressure at high temperatures. In fact, high pressures at high temperature are what transforms our soft, flaky graphite into hard-edged diamond. The transformation requires a huge amount of energy, because some carbon atoms have to jump long distances to create that perfect cube. |
|
|
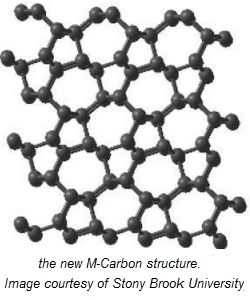
And now, between the two, we have ‘superhard graphite’. The ‘superhard graphite’ is the result of exposing graphite to diamond-creating high pressures; but doing so at (relatively very cold) room temperatures. The first appearance of this new form of carbon was in 1963, when two curious scientists called Aust and Drickamer first compressed graphite at room temperature. Like diamond, this new form of carbon was transparent and very hard. But in other ways this substance was unlike diamond or indeed, any other forms of carbon. In 2006, Dr Artem Oganov, professor of theoretical crystallography in the Department of Geosciences at Stony Brook University, NY, proposed a crystal model for ‘superhard graphite’ which substance he called M-Carbon. Assuming you have a specialized million-dollar laboratory at your disposal, producing ‘superhard graphite’ is simple. As Dr Oganov explains: "The experiment itself is striking: you compress black ultrasoft graphite, and then it suddenly turns into a colorless, transparent, super-hard and mysterious new form of carbon -- 'superhard graphite,'" The problem heretofore had been that the resolution of experimental data was too low to produce a convincing structural model for the end product. So after Dr Oganov’s model, there was a flood of papers suggesting possible structures for ‘superhard graphite’. To discriminate between these models, higher-resolution experimental data and additional theoretical insight were required. So Dr Oganov and his colleagues used a powerful simulation approach - recently adapted to solid materials - known as transition path sampling. Transition path sampling (TPS) is a method used in computer simulations of physical or chemical transitions from one stable state to another in types of event that occur too rarely to be observed on a computer timescale. Examples include protein ‘folding’ or some chemical reactions. Dr Oganov and his colleagues started with the assumption that creating the ‘superhard graphite’ crystal structure requires the expenditure of much less energy than that needed to create a perfect diamond cube. Which means that the carbon atoms which create the new bonds must already be relatively close together. Using the TPS approach Dr Oganov and his colleagues confirmed that his original model of M-Carbon is indeed the structure of ‘superhard graphite’. Their results have been published in the in Scientific Reports, a Nature journal (ref. 1), and can be viewed online. In the 2012 July edition of Scientific Reports, a group from Yale University led by Dr Lee published experimental data which confirmed for the first time through high-pressure experiments that the structure of cold-compressed graphite is indeed that of M-carbon. This paper can also be viewed online (ref. 2). The Yale group used a number of methods including long duration x-ray diffraction, Raman spectroscopy and optical techniques to verify their data. This experimental approach has shown that the transition from graphite to M-Carbon is extremely sluggish and takes a long time to reach equilibrium, which, the authors believe, may be an additional reason why the structure of superhard graphite remained a puzzle for the past half century. The experimental studies have also shown that although M-Carbon at the crystal level has much lower symmetry than diamond, it is so hard that it can actually damage the diamond itself, which no other compound is known to be able to do. Journal Reference: 1. Salah Eddine Boulfelfel, Artem R. Oganov and Stefano Leoni. Understanding the nature of “superhard graphite”. Scientific Reports, 2012, 2 (http://www.nature.com/srep/2012/120628/srep00471/full/srep00471.html) 2. Yuejian Wang, Joseph E. Panzik, Boris Kiefer, Kanani K. M. Lee. Crystal structure of graphite under room-temperature compression and decompression. Scientific Reports, 2012; 2 (http://www.nature.com/srep/2012/120719/srep00520/full/srep00520.html) | |
| _______________________________ | ||||
| Home | | | Shopping | | | Database |
© Biscuit Software 2004-2015
All rights reserved